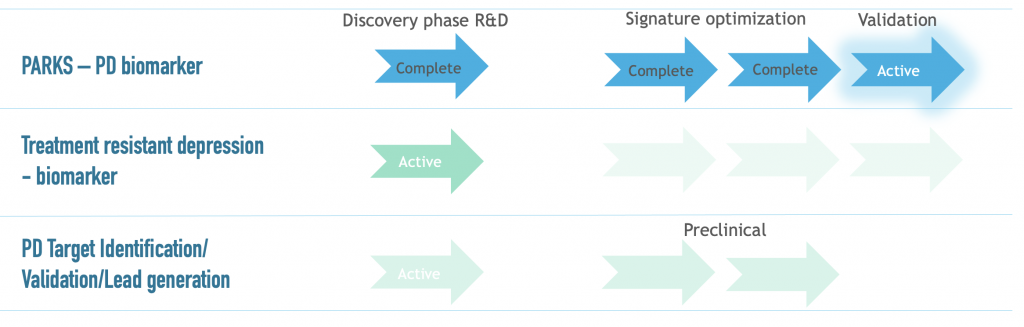
PARKS offers a diagnostic test that identifies Parkinson’s disease from a blood sample. Our signatures differentiate between Parkinson’s disease and non-Parkinsons’s movement disorders with overlapping symptoms.
Our goal is to improve these signatures in order to detect Parkinson’s at a stage before motor symptoms occur. In addition to assisting diagnosis, this technology can be used to screen patients for clinical trials, thereby reducing the risk for investment in new treatments.
We are interested in partnering with pharmaceutical companies leading clinical trials for Parkinson’s disease and with diagnostic service providers to expand the application of PARKS signatures to a broad user base.
PARKS signatures result from mass spectrometry-based study of a multi-condition cohort of patients and healthy donors from Southwest Finland. All participants have undergone a DaTScan and patients are monitored by UPDRS testing. PARKS signatures distinguish Parkinson’s disease from healthy or non-Parkinson’s movement disorders with an A.U.C. of >85%. We are currently validating these signatures using an independent, large cohort which includes prodromal cases and longitudinal sampling. In addition, we are partnered with an Asian hospital to test the accuracy of our signatures in demographics beyond Europe and the
We also investigate LRRK2-dependent disease mechanism. Now in early-stage R&D, we are following three novel targets with potential for drug discovery. In addition to Parkinson’s, we are identifying prognostic biomarkers for treatment-resistant depression.
PARKS is supported by the Michael J. Fox Foundation, PPMI, Business Finland, and the Academy of Finland.